

Objective Data Reveals Prevalence of Diverse Symptoms in HPV Vaccine-Unvaccinated Individuals
Providing Scientific Evidence to Support Improved Vaccination Rates
A research group led by Yurika Kawazoe from the Clinical Research Center at Nagasaki University Hospital, in collaboration with St. Marianna University School of Medicine, Shizuoka Kosei Hospital, and Kyushu University, has used large-scale real-world data from Japan to reveal that diverse symptoms similar to those reported after HPV vaccination occur at certain rates among unvaccinated Japanese adolescents aged 10-19. These research findings were published in the journal Vaccine on November 29, 2025. This study provides important baseline data for distinguishing whether specific symptoms are caused by HPV vaccination or are naturally occurring symptoms that already exist in the population when evaluating vaccine safety.
• In Japan, the active recommendation for HPV vaccination was suspended from 2013 to 2022, and the recovery of vaccination rates remains a public health challenge due to safety concerns.
• While previous reports indicated that “diverse symptoms reported after vaccination” also occur in unvaccinated individuals, these were based on questionnaire surveys, and verification using objective, large-scale data was needed.
• Using the VENUS research database (linking National Health Insurance claims data with vaccination records), we calculated the prevalence of diverse symptoms among unvaccinated males and females aged 10-19.
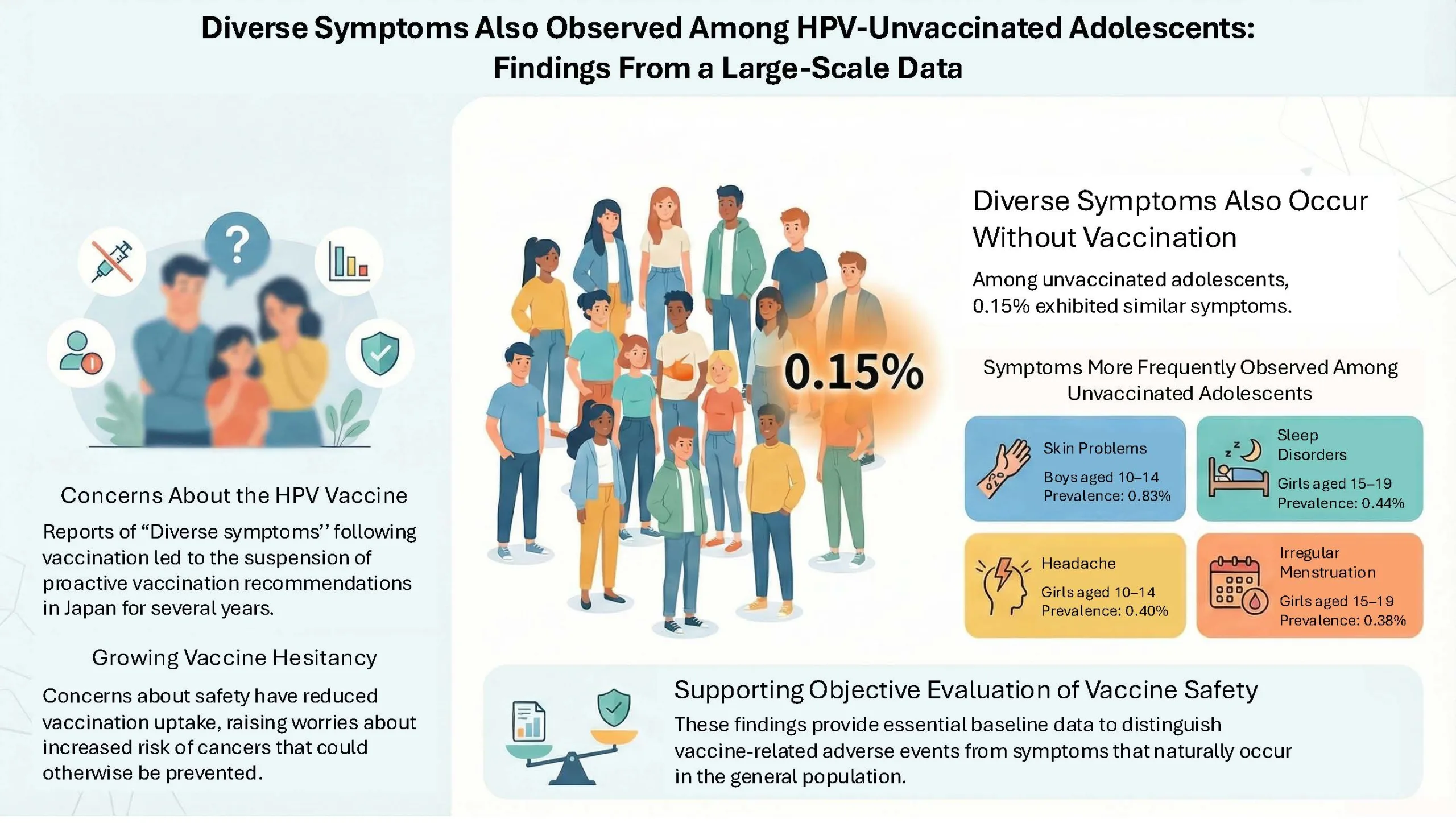
• The overall symptom reporting rate was 0.15%, with the most common symptoms being skin problems, sleep disorders, headaches, and menstrual irregularities.
• To accurately determine whether specific symptoms are attributable to vaccination, it is essential to know the baseline frequency of occurrence in unvaccinated populations. This study provides fundamental data for such comparison groups.
Background
While HPV vaccines have been proven to prevent HPV infections that cause cervical cancer, Japan suspended active vaccination recommendations in June 2013 following media reports of adverse events after vaccination. This measure led to a rapid decline in vaccination rates in Japan, raising serious public health concerns, including increased cervical dysplasia and cervical cancer among young women.
Although active recommendations resumed in April 2022, vaccination rate recovery has been slow, and achieving the WHO target of “90% vaccination among girls under 15 by 2030” is predicted to be difficult. Due to insufficient data on vaccine safety in Japan, hesitancy toward the vaccine continues among eligible individuals and their families.
While the Sobue Research Group previously reported that “diverse symptoms are observed in a certain number of unvaccinated individuals,” this was based on questionnaire surveys, and concerns were raised about potential data inaccuracy and bias.
Research & Results
In this study, we analyzed data from four municipalities between April 2017 and March 2022 using the VENUS research database, which corresponds to Japan’s Vaccine Safety Datalink (VSD). The study included 55,483 individuals aged 10-19 who were enrolled in National Health Insurance and had not received the HPV vaccine. We defined diverse symptoms as 27 symptom categories from previous literature that persisted for more than three months without identifiable underlying diseases. A consensus conference of three pediatricians was conducted to determine diverse symptoms.
The results revealed the following:
・The overall symptom reporting rate was 0.15%, which was 7.5 times higher than the Sobue Research Group’s report (0.02%)
・Females (ages 10-14: 0.17%, ages 15-19: 0.17%) showed slightly higher reporting rates than males (ages 10-14: 0.16%, ages 15-19: 0.09%)
・The most common symptoms were skin problems (males ages 10-14: 0.83%), sleep disorders (females ages 15-19: 0.44%), headaches (females ages 10-14: 0.40%), and menstrual irregularities (females ages 15-19: 0.38%)
・No significant variation in reporting rates was observed across municipalities or during the COVID-19 pandemic period
The symptom reporting rates obtained in this study, together with the results from the Sobue Research Group, will serve as important baseline data for distinguishing between true vaccine-related adverse events and naturally occurring symptoms in future vaccine safety surveillance.
Future Prospects
The unvaccinated population’s symptom reporting rate data obtained in this study will serve as an essential reference point for conducting further epidemiological research on HPV vaccine safety. Additionally, this study demonstrates the methodology and potential for establishing a continuous vaccine safety monitoring system in Japan. Moving forward, evaluation using larger-scale data is needed to improve the accuracy of assessing rare symptoms. Furthermore, comparative safety studies between vaccinated and unvaccinated groups using large-scale, objective data are required to demonstrate HPV vaccine safety to the Japanese public.
The results of this study are expected to contribute to restoring confidence in HPV vaccine safety and improving vaccination rates in Japan.
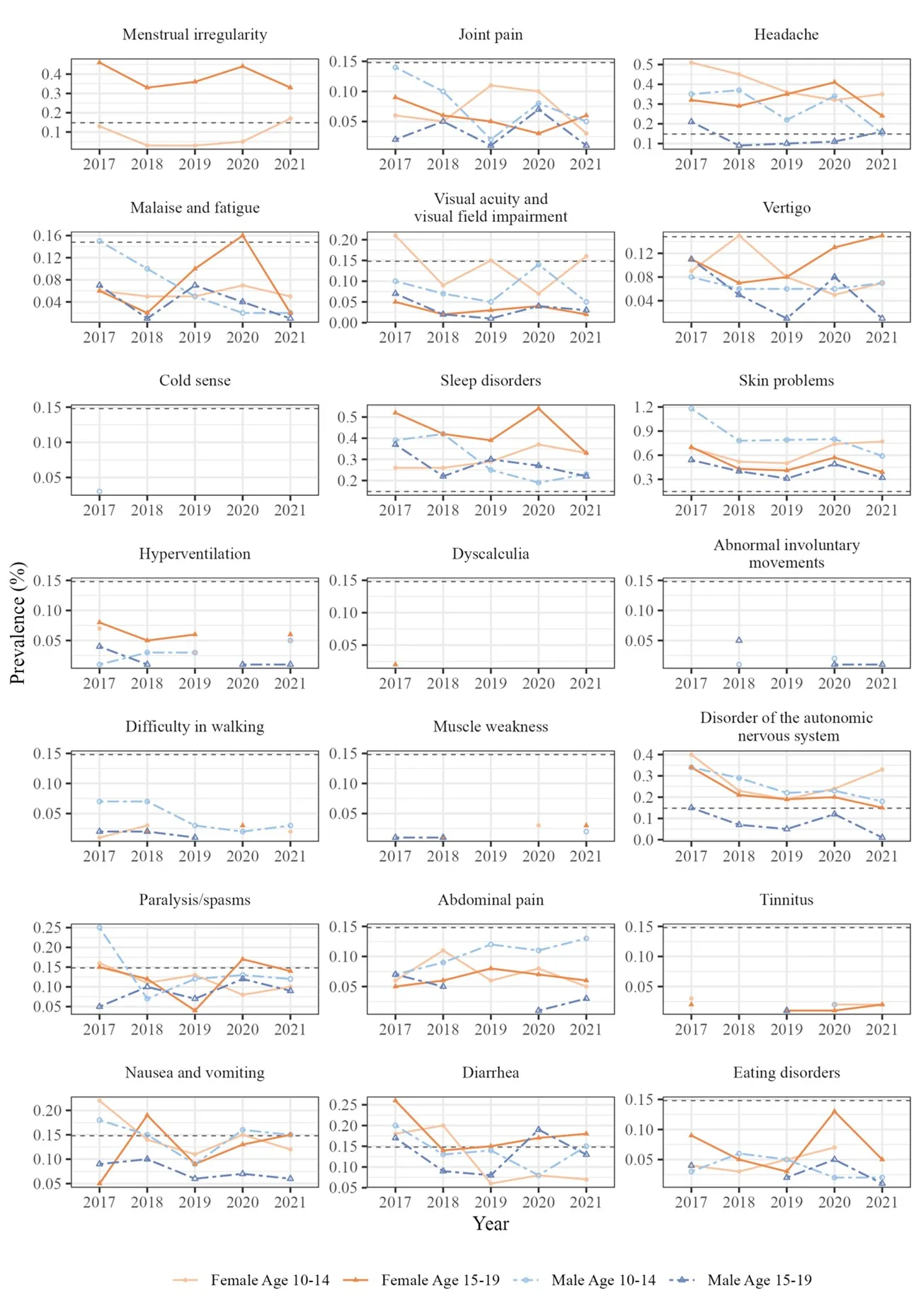
Figure 3. Prevalence of the diverse symptoms among HPV-unvaccinated persons.
Journal: Vaccine
Title: Prevalence of HPV vaccine-associated symptoms in unvaccinated Japanese adolescents: A descriptive study from the VENUS study database
Authors: Yurika Kawazoe, Tomohiro Katsuta, Toshihiro Tanaka, Yukitsugu Nakamura, Shuntaro Sato, Megumi Maeda, Futoshi Oda, Haruhisa Fukuda
Publication Date: November 29, 2025 (JST), Vaccine 70 (2026) 128020, by Elsevier
DOI: https://doi.org/10.1016/j.vaccine.2025.128020
Yurika Kawazoe, Clinical Research Center, Nagasaki University Hospital
email: kawazoe-y*nagasaki-u.ac.jp
When you send the email, please replace * with @.
This research was supported by grants from the Japan Agency for Medical Research and Development (AMED) (JP21nf0101635) and the Japan Science and Technology Agency (JST) FOREST Program (JPMJFR205J).
For more details, please see the full article published in Vaccine.