

Early determination of the dorsal-ventral axis in endochondral ossification in mice
An international research team led by Professor Yuki Matsushita from the Nagasaki University Graduate School of Biomedical Sciences and Dr.Noriaki Ono of the University of Texas in the United States, has discovered an important mechanism involved in bone development and growth—specifically, the origin of the cells responsible for forming the dorsal (back) side of the limb skeleton (arms and legs)—through an international collaborative study.
The results of this research were published on November 29, 2025, in the U.S.-based international academic journal Journal of Bone and Mineral Research.
In the development of long bones such as those in the arms and legs, it has been known that the mesenchymal condensations※1 that first appear during the fetal stage and the subsequent stage, the cartilage primordia※2, serve as the basis for almost all bones. However, it had not been well understood how these structures ultimately shape mature bones.
By successfully visualizing and tracking the fate of cells in specific regions of the mesenchymal condensations and cartilage primordia, the researchers identified the origin of the cells that later form the dorsal (back) side of the limb bones (arms and legs).
These findings are expected to contribute to a better understanding of the pathology of rare congenital disorders involving skeletal abnormalities and to the development of new therapeutic approaches.
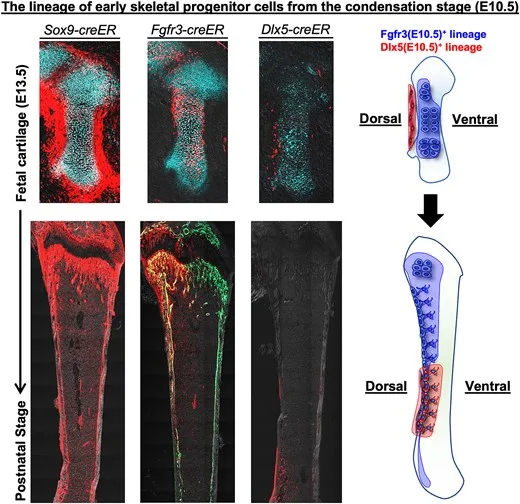
Endochondral ossification is a highly coordinated process involving distinct progenitor cell populations within the mesenchymal condensation and subsequent cartilage anlage and perichondrium, all of which drive skeletal formation. Cell-type specific lineage tracing conducted to understand fetal bone development has revealed various fates of early skeletal cells. However, the underlying continuous and precise cellular dynamics of fetal skeletal cells, particularly along the dorsoventral axis, remain unclear. Here, we show that spatiotemporally specific skeletal progenitor cells in the early developmental stage contribute to the dorsal-ventral axis in a manner that is strictly determined during initial developmental stages. Lineage-tracing experiments using Fgfr3-creER and Dlx5-creER lines revealed that Fgfr3+ cells in mesenchymal condensation exclusively contributed to hypertrophic chondrocytes and the dorsal side of the resting and proliferating zones within the cartilage anlage. These cells made dorsal-restricted contributions to skeletal development, including growth plate chondrocytes, trabecular and cortical osteoblasts, and bone marrow stromal cells. Functional ablation of Fgfr3+ cells using the Rosa26iDTA (inducible diphtheria toxin fragment A) allele during the mesenchymal condensation stage caused severe disruption in long-bone development, underscoring its indispensable role in initiating skeletal growth. Collectively, these findings suggest that the condensation stage is pivotal for the formation of skeletal progenitors and dorsoventral patterning during bone development. Understanding these mechanisms will provide insight into skeletal growth disorders and therapeutic strategies for bone regeneration.
In Japan and around the world, there are many pediatric rare diseases whose causes remain unknown, and many of these are associated with abnormalities in bone development. Accurately understanding the processes of bone development is therefore critically important, as it may lead to the elucidation of these diseases. It has been known that during bone development, mesenchymal condensations that first appear in the fetal stage and the subsequent stage, known as cartilage primordia, serve as the origin of almost all bones. However, it has not been well understood how these structures ultimately give rise to mature bones—for example, which specific regions of a mesenchymal condensation later become particular parts of a bone.
In this study, the researchers first focused on the early stages of bone development. By performing single-cell RNA sequencing※4 on cells isolated from mesenchymal condensations in mouse embryos, they revealed the diversity of cells present at the earliest stages of bone development at single-cell resolution. Furthermore, by applying data science approaches, they discovered that cells within mesenchymal condensations—previously thought to be homogeneous—are in fact heterogeneous and can be divided into cells that strongly express the gene Fgfr3※5 and those that do not. Histological analyses also showed that Fgfr3 is localized in cells situated in a more central region of the mesenchymal condensations.
Next, to determine how these centrally located Fgfr3-expressing cells within mesenchymal condensations contribute to bone formation from the early fetal stages through adulthood, the researchers visualized Fgfr3-positive cells using red fluorescent molecules and successfully tracked their fate using a technique called “cell lineage tracing.” The results demonstrated that Fgfr3-expressing mesenchymal condensation cells present during the fetal stage selectively contribute after birth to the cells that form the dorsal (back) side of adult limb bones. Moreover, when cell death was selectively induced in these Fgfr3-expressing mesenchymal condensation cells, longitudinal bone growth was almost completely abolished, revealing that Fgfr3-expressing cells play a critical role in bone growth.
These findings indicate that the origin of the cells composing adult bones is strictly predetermined before birth, and this study newly elucidates how the front and back (ventral and dorsal aspects) of bones are established during development (see schematic overview below).
Based on these findings, a detailed elucidation of the processes of bone development is expected to contribute to a better understanding of the pathology of congenital disorders accompanied by skeletal abnormalities, as well as to the development of new therapeutic approaches.
Graphical Abstract
※1 Mesenchymal condensation:
A cluster of cells observed in the early stages of vertebrate bone formation that serves as the precursor of bone and the origin of the future skeleton.
※2 Cartilage primordium:
A structure that represents the stage following mesenchymal condensation, formed when undifferentiated mesenchymal cells differentiate into chondrocytes; it likewise serves as a precursor of the future skeleton.
※3 Cell lineage tracing:
A histological technique used to track the fate of a targeted population of cells over time.
※4 Single-cell RNA sequencing analysis:
A method for comprehensively analyzing gene expression at the level of individual cells.
※5 Fgfr3 (Fibroblast Growth Factor Receptor 3):
A transmembrane receptor protein that binds members of the FGF family; it is expressed in chondrocytes of the cartilage primordium during the early stages of bone development.
Journal: Journal of Bone and Mineral Research 40(12): 1385-1396, 2025
Title: Early determination of the dorsal-ventral axis in endochondral ossification in mice
Authors: Sixun Wu , Hirotaka Matsumoto , Jumpei Morita , Mina Yamabe , Azumi Noguchi , Shinsuke Ohba , Noriaki Ono , Yuki Matsushita
DOI: 10.1093/jbmr/zjaf086
For more details, please refer to the full article published in the Journal of Bone and Mineral Research.